In a previous post I introduced some of the basics of what I do as part of my DPhil (the Oxford term for a PhD: actually more English as the term PhD, I believe, originates in America.) In this post I will cover a bit more about the lasers we use to perform these experiments.
I mentioned two concepts in the last post: Lasers and Plasmas. We use lasers to create a plasma in the lab, but what exactly is a laser and why can it be used to make plasmas?
LASERs – “A solution looking for a problem”*
When they were first discovered no one could think of a use for them. However lasers are now ubiquitous in our lives: from DVD or Blu-Ray players to barcode readers, medical applications (eye surgery, skin treatments) to science research, lasers have made a big impact.
Laser is actually an acronym which stands for Light Amplification by Stimulated Emission of Radiation. Effectively a laser is a very special source of light that has specific properties:
- It is coherent – this allows the light to be focussed very tightly,
- Many can be made with only a single colour of light (wavelength),
- It is highly directional – unlike a normal light, you tend not to be able to see a laser if you are side on to the direction the light is moving,
- It can be amplified to contain a large amount of energy, in a short amount of time.
The light in a laser comes from atoms within the material. Atoms are made up of a positive nucleus around which negative electrons move. The electrons are arranged into certain positions (which we call orbits) around the nucleus, and they want to be as close to the nucleus as possible.
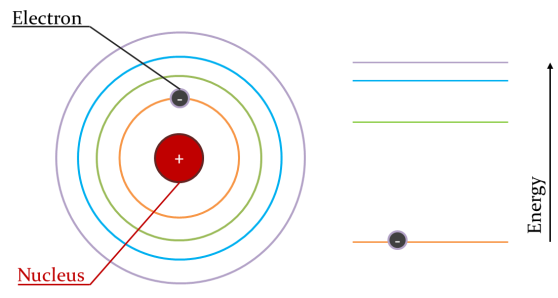
We can see the nucleus with the electron orbiting. The orbitals are shown in different colours increasing in distance from the nucleus. To the side we see these orbitals arranged in terms of energy. The electron is sitting in the lowest energy level.
An electron can absorb light and move further from the nucleus, gaining in energy. Now the electron is in a higher energy level than it wants to be, so it can emit light and drop back down, we call this Spontaneous Emission. In certain cases, the electron can be made to emit energy if light comes along that has the same amount of energy as the electron wants to lose. In this case twice the amount of light comes out – the original light and the light from the electron – with the same energy and both are coherent. This is called Stimulated Emission and is the basis for a laser working! We call these individual pieces of light ‘photons’.
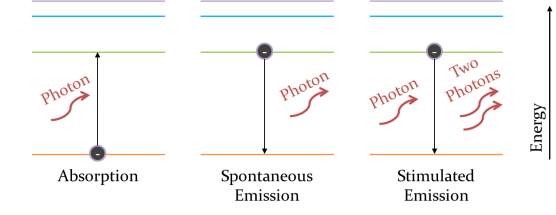
In Absorption, a photon of light comes in and is absorbed by the electron. The electron moves further from the nucleus, increasing in energy. In Spontaneous Emission, an excited electron decides it doesn’t want so much energy, so releases a photon and drops back down to a lower energy orbit. In Stimulated Emission, a photon with energy equal to the separation of the two energy orbits stimulates the electron to emit a photon and drop down.
*This appears to be what was said about lasers when they were first discovered, however I can’t find the original quote, or who even said it!
The Physical “Machine”
Now that we know how light and matter can interact, we can put together an actual laser.
We need:
- A source of photons,
- A way of making sure that once one photon of light is released, it goes on to stimulate more photons being released.
This, is turns out, is solved using special crystals and mirrors.
We place a crystal between two mirrors: we call this a cavity. Around the crystal we place lamps, know as flash lamps. These are just like standard bulbs, and they are designed to repeatedly flash on and off around the crystal. As they emit light, the electrons in the crystal absorb this and raise their energy level. Some electrons will then decide to drop down and release a photon of energy (the squiggly lines in the photo below.) This light is released in random directions, however if one happens to travel along the direction that it hits one of the mirrors and is reflected back into the crystal, this can stimulate another electron to drop down, and now we get 2 photons: doubling the light! These then reflect off the other mirror and return to the crystal where these two photons stimulate two electrons to emit and now we get 4 photons. This carries on until we have a vary high number of photons!
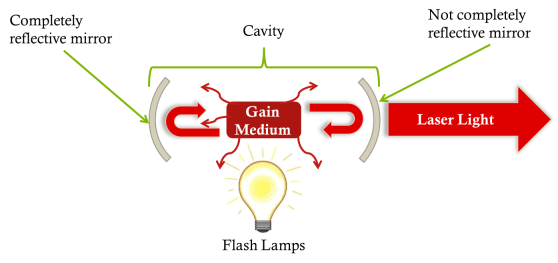
Showing a schematic of a laser. The squiggly red arrows are spontaneously emitted photons, the heavy red arrows are photons that have been emitted by stimulated emission.
One of the mirrors is not completely reflective, so it allows some light through. As the amount of light in the cavity increases, so too does the output. This output is the laser light!
A laser can be used to create a plasma
As I mentioned in my previous post, and earlier, we use lasers to create plasmas that we are interested in studying. But how does this work?
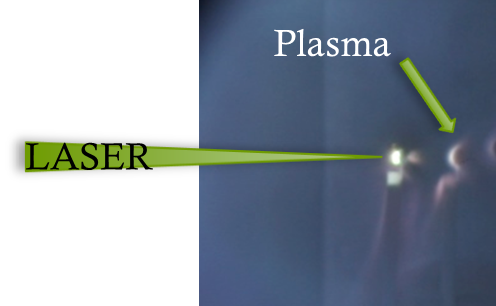
An experiment showing the light emitted from a plasma that was formed when the laser was fired onto a carbon rod (much like a pencil lead).
A plasma is formed by separating electrons from their atoms. This takes a lot of energy, and laser light contains a lot of energy. When the laser hits onto a piece of material, the light is absorbed by the electrons in the atom. However; whereas before when the flash lamp caused an electron to increase in energy but stay in an orbital of the atom, here the energy is high enough that the electron can be kicked out of the atom completely. When an electron leaves its parent atom, this is known as ionisation. As the laser is so intense (there is a lot of light in a short amount of time and area), many electrons absorb the energy, so many atoms can be ionised multiple times.
These electrons are now have a lot of energy, and are hot: they move around very fast. Whereas the atoms before were neutral and happy, now the electrons (negatively charged) are moving separately from the remaining ions (positively charged). As there is a separation of the charge, electric forces can act between the two. In this way the ions can also gain energy from the electrons. (This is a simplified view of how energy is given to the electrons and ions as there can be many other processes. However it is sufficient to get a general idea of what is happening.) Voilà: a plasma is formed!
What Next?
We now know how lasers work, and that we can produce plasmas with them. But, how can we use lasers to recreate a star in a lab? Tune in next time to find out!